Homework 2
of Introduction to Structural Biology
1.
Ans:
I discovered that the frequencies of amino acid replacements has something
to do with "the genetic code",
for example: Ser is high frequency replaced by Asn showed in the table.
The genetic code for Asn is AAU
AAC,
and for Ser is AGU
AGC.
So dose Ile and Val. So it is a significant proof. On the other hand, dose
the chemical nature of the amino acid residues involved? My answer is "Yes"!
The table is not so regulatory, the genetic code dose not mean everything!
Like the table shows, the R group has an important role.If the differences
between two homologous species are examined, a general tendency is observed
for chemically similar amino acid residues to be found at the same position.
For example: Ala-Ser is higher than Ala-Pro .The more similar the number
of carbon on R group, the higher frequency of replacement.
2. Why is it important to establish
protein sequence homologies?
Ans:
Primary structure determines all higher levels of structure.The Three-dimensional
Structure of Proteins is More Highly Conserved than the Primary Structure.
So if the primary structure, ie.the a.a. sequence, has high homology, it
may have similar 3D structure. We may predict they have the similar
function!
3.(a)
Define the term "chiral centre" as applied to amino acids.
Ans:Generally,
the alpha-carbon is the chiral center! The four different groups attached
to alpha-carbon, it is said to be asymmetric.
Two possible configuration for the alpha-carbon constitude nonidentical
mirror image. Apply the "D,L system".
(b)
Which amino acids have chiral centers that are not alpha-carbon atoms?
Ans:Thr
and Ile have two chiral centers.
 Thr
Thr  Ile
Ile
They
have another chiral center in R-group, not alpha-carbon. So they have two
chiral centers.
(c)
Which amino acid is this one ? Is it a D-amino acid ?
Ans:Asparagine(Asn),
and it is a L-a.a.
Because it obey CORN Rule.
 L-Asn
L-Asn
4. (a) Why the gauche(+)
is the most abundant conformation of chi1?
Ans:
The most abundant conformation is gauche(+) in which the gamma side chain
atom is opposite to the residue's main chain carbonyl group when viewed
along the Cbeta-Calpha bond.
(b) What
is the gauche(+) conformation ?
Ans:

(c)
Why aliphatic amino acids which are bifurcated at Cb, ie valine and isoleucine,
do not adopt the trans conformation very often ?
Ans:Aliphatic
amino acids which are bifurcated at Cbeta, ie valine and isoleucine, do
not adopt the trans conformation very often as this involves one of the
Cgamma atoms being in the unfavourable gauche(-)'position'.In general,
side chains tend to adopt the same three torsion angles (+/-60 and 180
degrees) about chi2 since these correspond to staggered conformations.
However, for residues with an sp2 hydridised gamma atom such as Phe, Tyr,
etc., chi2 rarely equals 180 degrees because this would involve an eclipsed
conformation. For these side chains the chi2 angle is usually close to
+/-90 degrees as this minimises close contacts. For residues such as Asp
and Asn the chi2 angles are strongly influenced by the hydrogen bonding
capacity of the side chain and its environment. Consequently, these residues
adopt a wide range of chi2 angles.
5.(a ) Explain the meaning
of each colored regions?
Ans:
The red regions correspond to conformations where there are no steric clashes,
ie these are the allowed regions namely the alpha-helical and beta-sheet
conformations. The yellow areas show the allowed regions if slightly shorter
van der Waals radi are used in the calculation, ie the atoms are allowed
to come a little closer together. This brings out an additional region
which corresponds to the left-handed alpha-helix.
(b)
Which two groups or atoms are involved for the steric hindrance in the
disallowed regions of this plot?
Ans:
N-Calpha
and Calpha-Co

(c) Which
amino acid can adopt phi and psi angles in all regions? Why?
Ans:Gly,
because Glycine has no side chain, and has no chiral center. May be take
it for "D-form" or "L-form" amino acid. And therefore can adopt phi and
psi angles in all four quadrants of the Ramachandran plot. Hence it frequently
occurs in turn regions of proteins where any other residue would be sterically
hindered.
6.
(a)
Why is the C-N bond length of the peptide 10% shorter than that found in
usual C-N amine bonds?
Asn:
Because amino acid in
neutral solution(pH=7.0),the carbonyl group exists as -COO-
and the amino group as -NH3+ , resulting amino acid
contain one positive and one negative charge, it is a neutral molecular
called a "zwitterion".
And it may rasonance! C-N bond, ie. the peptide bond have
partial double bond character! So it shorter
than usual C-N single (amide) bonds.
(b)
Why the peptide bond nearly always has the trans configuration ? Which
amino acid is found in the cis configuration more frequently than other
amino acids ? Why?
Ans:The
peptide bond nearly always has the trans configuration since it is more
favourable than cis, which is sometimes found to occur with proline
residues.
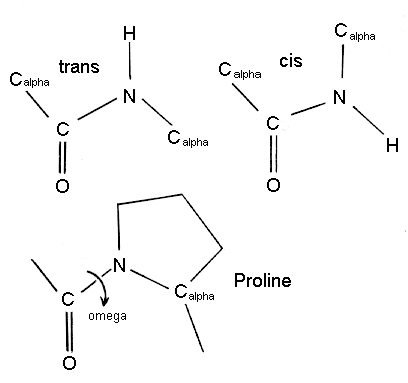
Steric hindrance between the functional
groups attached to the Calpha atoms will be greater in the cis configuration.
However for proline residues, the cyclic nature of the side chain means
that both cis and trans configurations have more equivalent energies. Thus
proline is found in the cis configuration
more frequently than other amino acids. The omega torsion angle of proline
will be close to zero degrees for the cis configuration, or most often,
180 degrees for the trans configuration.
7. What factors cause a-helices in globular proteins
to distort ?
Ans:
These
distortions arise from several factors including:
1.The packing of buried helices against other secondary
structure elements in the core of the protein.
2.Proline residues induce distortions of around
20 degrees in the direction of the helix axis. This is because proline
cannot form a regular alpha-helix due to steric hindrance arising from
its cyclic side chain which also blocks the main chain N atom and chemically
prevents it forming a hydrogen bond. Janet Thornton has shown that proline
causes two H-bonds in the helix to be broken since the NH group of thefollowing
residue is also prevented from forming a good hydrogen bond. Helices
containing proline are usually long perhaps because shorter helices would
be destabilised by the presence of a proline residue too much.
Proline occurs more commonly in extended regions of polypeptide. Proline
is a alpha-helix teminator!
3.Solvent. Exposed helices are often bent away from
the solvent region. This is because the exposed
C=O groups tend to point towards solvent to
maximise their H-bonding capacity, ie tend to form
H-bonds to solvent as well as N-H groups.
This gives rise to a bend in the helix axis.
4.3(10)-Helices. Strictly, these form a distinct class of helix
but they are always short and frequently occur
at the termini of regular alpha-helices. The name 3(10) arises
because there are three residues per turn and ten atoms enclosed in a ring
formed by each hydrogen bond (note the hydrogen atom is included in
this count). There are main chain hydrogen bonds between residues separated
by three residues along the chain (ie O(i) to N(i+3)). In this nomenclature
the Pauling-Corey alpha-helix is a 3.6(13)-helix. The
dipoles of the 3(10)-helix are not so well aligned as in the alpha-helix,
ie it is a less stable structure and side chain packing is
less favourable.
1998/10/25 Jiun-Ming Wu
 Thr
Thr  Ile
Ile
 L-Asn
L-Asn




