(1) ID number : 1WRT
(2) Authors : D. ZHAO, Z. ZHENG
(3) Resolution : Not applicable
(4) 108 residues in one monomer

 Trp repressor itself
Trp repressor itself

| Apo form (1) ID number : 1WRT (2) Authors : D. ZHAO, Z. ZHENG (3) Resolution : Not applicable (4) 108 residues in one monomer |
 Trp repressor complexed with operator
Trp repressor complexed with operator
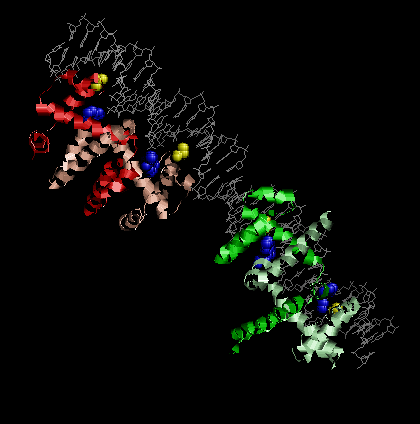 | (1) ID number : 1 TRO (2) Authors : Z. OTWINOWSKI, R. G. ZHANG, P. B. SIGLER (3) Resolution : 1.9 Å (4) How many cofactors in whole complex --- 4 cofactors (trp) |
 Molecular mechanism
Molecular mechanism
Structural changes in the Trp repressor induced by binding of the cofactor, L-tryptophan. Where the structure is altered by binding of l-tryptophan, the shaded model is that without the ligand. Helices A - F are from one polypeptide chain of the dimer, a - f are from the other. The ligand bound to each of the two sites of the dimer is shown as a skeletal model. The ligand binds between helices B, C, and E of each half of the dimeric protein. In doing so, it pushes helices D and E of the helix-turn-helix motif away from the center of the molecule and places the two equivalent recognition helices E and e the correct distance apart to bind in adjacent major groove of normal double-helical DNA. The binding affinity for DNA is increased 1000-fold by the presence of the ligand. ( From R. G. Zhang et al., Nature 327:591-597, 1987. )
![]()
(a) Show the DNA sequences of the Arc operator.
2.
Transcription of the ant gene during lytic growth of bacteriophage P22 is regulated by the cooperative binding of two Arc repressor dimers to a 21-base-pair operator site. Please find the co-crystal structure of this Arc tetramer-operator complex from PDB.
5' ATAGT AGAGT GCTTC TATCA T 3'
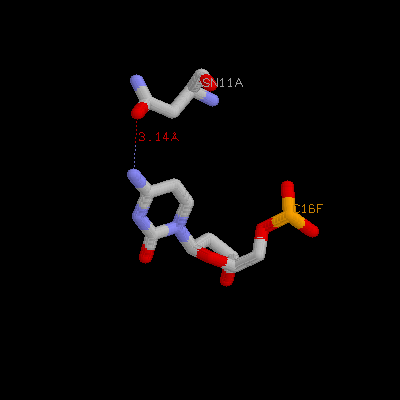
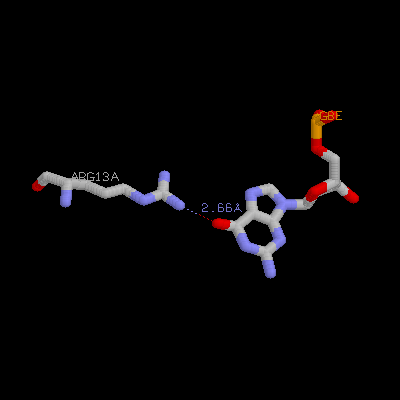
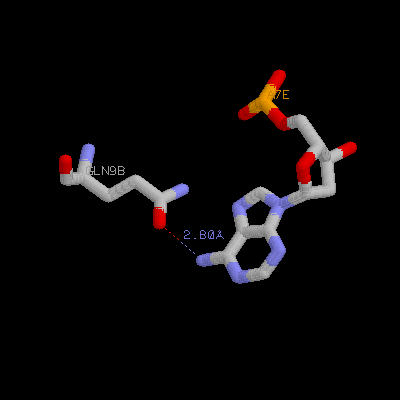
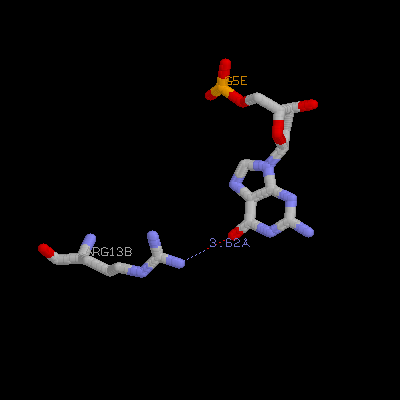
(b) Show three side chains (gln9, asn11, arg13) from one monomer and corresponding DNA bases of them on your home page. Color three side chain with different color. Measure the distance between possible hydrogen bonds (at least three)
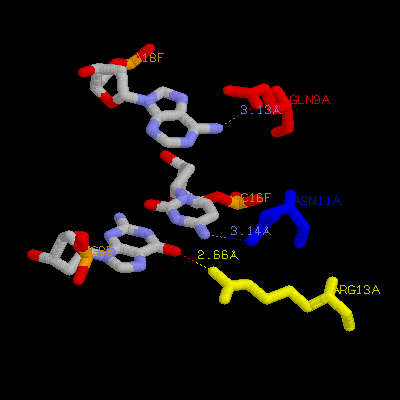
Gln9a ---- A18F
Asn11a ---- C16f
Arg13a ---- G8e