|
 |
 |
 |
 |
Publication Year: 2000
Visited: 48
|
484
Her-2-Neu (HER2) Overexpression in Locally
Advanced Breast Cancer (LABC) as Measured by Quantitative
Immunohistochemical (IHC) Analysis. C Wallace, S Day, A
Kallab, A Jillella, K Yeh, R Dalton, Medical Coll of Georgia,
Augusta, GA.
Although treatment with a recombinant humanized monoclonal
antibody (rhuMAb HER2, transtzumab) to the HER2 receptor has been
shown to be beneficial in women with metastatic disease whose tumors
overexpress HER2, the role of such therapy in the adjuvant or
neoadjuvant setting has not been defined. We sought to determine the
potential efficacy of such neoadjuvant therapy in women with LABC by
retrospectively testing paraffin-embedded specimens from primary
tumors with a commercially available quantitiative
immunohistochemical test for overexpression of HER2 (HercepTest,
Dako). 74 women with clinical or pathologic T3NOMO, T3 N1-2MO, or
T4N1-2MO tumors were identified through the tumor registry.
Sufficient material was available for testing in 61 women. Paraffin
blocks from primary tumor were then stained. Intensity of
immunostaing was scored 0-1+ (negative) or 2-3+ (positive) according
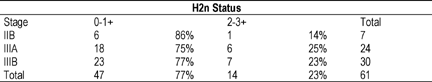
the guidelines and using internal controls. Patients were group into
Stages (IIB, IIIA, or IIIB) and by immunohistochemical staining
(negative 0-1+, or positive 2-3+). Overall, 23% (14/61) tumors
overexpressed HER2 oncogene as defined by 2-3+ immunostaining. This
did not vary by stage. [table] Overexpression of HER2 does not
appear to be more common in LABC than has been previously reported
in primary or metastatic tumors. It appears unlikely that the HER2
oncogene is somehow uniquely involved in the pathogenesis of LABC
and biological therapy directed at H2n is therefore unlikely to be
more or less useful in such patients.
|
