

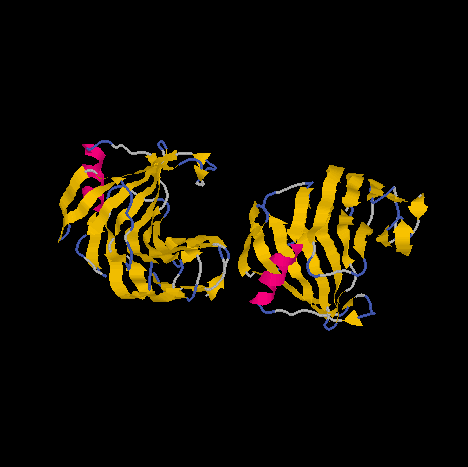 | Fig1. Ribbon representation of the XYNII molecule showing an alpha-helix and beta-strands. The structure is reminiscent of the shape of a 'right hand'. This protein has approximated dimensions of 32*34*42 Angstroms. |
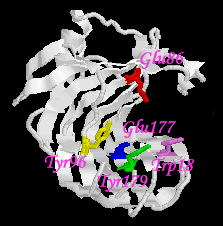 | Fig 2. The active site cleft in pH 5.0. |
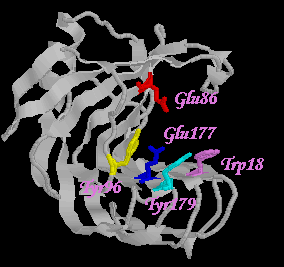 | Fig 3. The active site cleft in pH 6.5. |