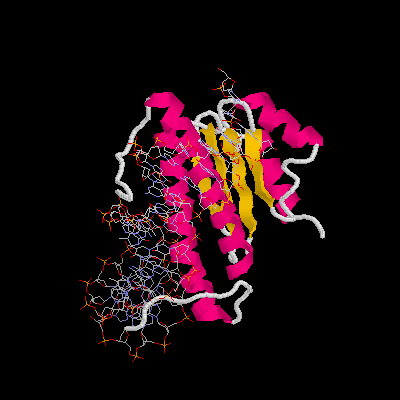Problem 1:
Here is protein X (header). Download and open it with RASMOL, and answer the following questions:
1.How many chains are there? What are the chain designatores (.i.e., the names of the chain)?
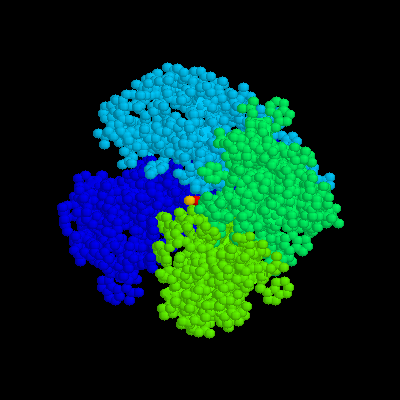
Ans: There 4 peptide chains in X.pdb, and their chain designatores are A, B, C and D in Rasmol.
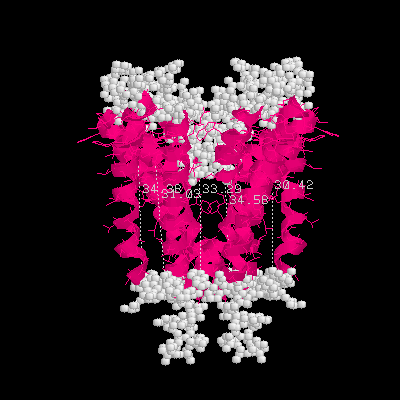
2.It is known that almost all helices are buried inside the membrane, while the sides are exposed in extracellular and intracellular parts. Use this information to estimate the thickness of the membrane.
Ans: Use Rasmol's picking distance function to get the distance of two terminals of each alpha helix segment, the thickness of membrane is estimated as 30 to 35 angstroms.
3.There are three metal ions, what are they?
Ans: They are potassium ions, labeled as 1E.K, 2E.K, 3E.K in Rasmol.
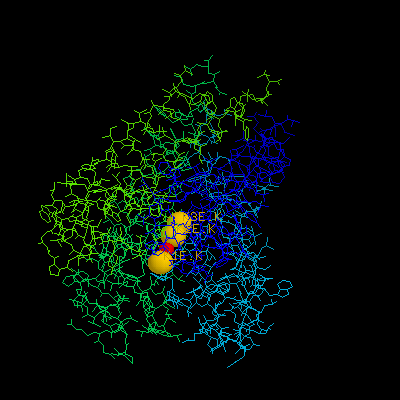
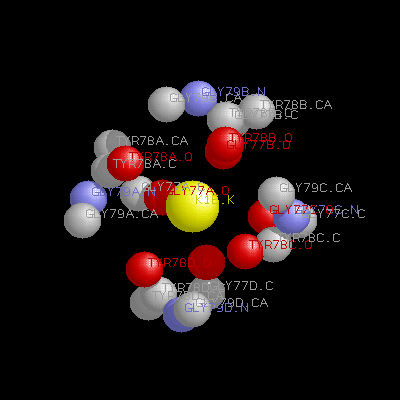
4.What are the residues, excluding water and metal ions, that are within 4.5 Å of the first metal ion?
(All the above answer should come with rasmol gif figures)
Ans: Gly77, TYR78 and GLY79 are whithin 4.5 Å of the first meta ion E1.K, for all 4 chains (A, B, C and D) in x.pdb.
Problem 2:
Find the paper where the authors published the structure of protein X mentioned in problem 1. Give the name of the Journal
and the abstract.
Ans: The paper is:
Science 1998 Apr 3;280(5360):69-77
The structure of the potassium channel: molecular basis of K+ conduction and selectivity.
Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R
Laboratory of Molecular Neurobiology and Biophysics and the Howard Hughes Medical Institute, Rockefeller University, 1230
York Avenue, New York, NY 10021, USA.
Abstract:
The potassium channel from Streptomyces lividans is an integral membrane protein with sequence similarity to all known K+
channels, particularly in the pore region. X-ray analysis with data to 3.2 angstroms reveals that four identical subunits create an
inverted teepee, or cone, cradling the selectivity filter of the pore in its outer end. The narrow selectivity filter is only 12
angstroms long, whereas the remainder of the pore is wider and lined with hydrophobic amino acids. A large water-filled
cavity and helix dipoles are positioned so as to overcome electrostatic destabilization of an ion in the pore at the center of the
bilayer. Main chain carbonyl oxygen atoms from the K+ channel signature sequence line the selectivity filter, which is held
open by structural constraints to coordinate K+ ions but not smaller Na+ ions. The selectivity filter contains two K+ ions about
7.5 angstroms apart. This configuration promotes ion conduction by exploiting electrostatic repulsive forces to overcome
attractive forces between K+ ions and the selectivity filter. The architecture of the pore establishes the physical principles
underlying selective K+ conduction.
Comments:
Science 1998 Apr 3;280(5360):56-7
Science 1998 Aug 14;281(5379):883
Science 1998 Aug 21;281(5380):1146
Probelm 3:
The human serum response factor is a transcription factor belonging to the MADS domain protein family with members
characterized from the plant and animal kingdoms. The X-ray crystal structure of the serum response factor core in a
specific-recognition DNA complex shows that the functions of DNA binding, dimerization and accessory-factor interaction are
compactly integrated into a novel protein unit. Please find the its PDB file in Protein Data Bank.
1.What is its PDB ID #?
Ans: Its PDB ID is 1SRS.
2.How many helices and beta-sheets in one protein chain?
Ans: There are 2 helices and 2 beta-sheets in one protein chain (total 4 helices and 4 beta-sheets for 2 protein chains).
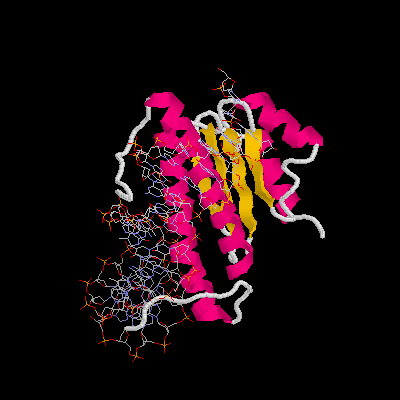
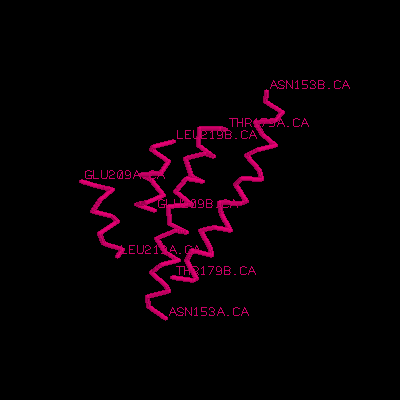
3.Please define the starting and ending residue of the helix. (ex. Ala25 -> Gly34)
Ans: (Glu209 -> Leu219) and (Asn153 -> Thr179).
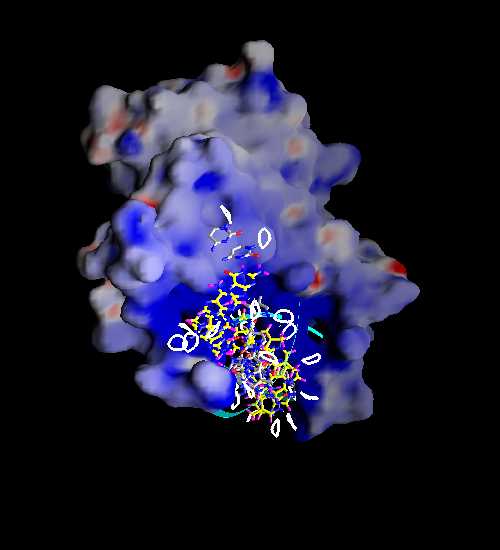
4.Find the model of molecular surface colored by electrostatic potential from Graphical Representation and Analysis of Structure Server at Columbia university. Put this picture in your webpage.
Ans: (http://trantor.bioc.columbia.edu/GRASS/data/pictures/1srs.p.jpg)