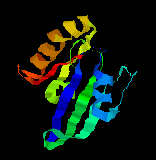 Ras protein
Ras protein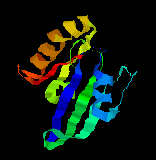 Ras protein
Ras proteinThe Ras proteins belong to the Ras superfamily of monomeric GTPases, which also contains two other subfamilies:(1)the Rho and Rac protein,involved in relaying signals from cell-surface receptors to the actin cytoskeleton,and(2) the Rab family,involoved in regulating the traffic of intracellular transport vesicles.Like almost all of these monomeric GTPases,the Ras proteins contain a covalently attached prenyl group that help anchor the protein to a membrane,in this case to the cytoplasmic face of the plasma membrane where the protein function.
The Ras protein help relay signals from receptor tyrosine kinases to the nucleus to stimulate cell proliferationor differentiation.If Ras function is inhibited by the microinjection of either neutralizing anti-Ras antibodies or dominant-negative mutant forms of Ras,the cell proliferation or differentiation responses normally induced by activated receptor tyrosine kinases do not occur;conversely,if a hyperactive mutant Ras protein is introduced into a cell,the effect on proliferation or differentiation is usually the same as that introduced by the binding of ligand to the cell-surface receptors.In fact,Ras proteins were first discovered as the hyperactive products of mutant ras genes,which promote cancer by disrupting the normal controls on cell proliferation and differentiation: about 30% of human cancers have such mutations in a ras gene.
Like the other monomeric GTPases and the trimeric G proteins,Ras proteins function as switches,cycling between two distinct conformational state-active when GTP is bound (Fig 1) and inactive when GDP is bound.Ras hydrolyzes GTP at least 100 times more slowly than the a subunit of the stimulatory trimeric G protein Gs,and because GTP is present in the cytosol at 10 times higher concentration than GDP,this means that in the absence of other influences,once GTP is bound,Ras would remain constantly active.In the cell.however,two classes of signaling proteins regulate Ras activity by influencing the transition between the active and inactive states.GTPase-activating proteins(GAPs)increase the rate of hydrolysis of bound GTP by Ras,thereby inactivating it.These negative regulators are counteracted by guanine nucleotide releasing proteins(GNRPs),which promote the exchange of bound nucleotide by stimulating the loss of GDP and the subseguent uptake of GTP from the cytosol;they therefore tend to activate Ras(Fig 2).In principle,therefore,receptor tyrosine kinases could activate Ras either by activating a GNRP or by inhibiting a GAP.Activated receptor tyrosine kinases bind GAPs directly,as we noted early,and bind GNRPs only indirectly,as we discuss below.Nonetheless,it is the indirect coupling to GNRPs that is usually responsible for driving Ras into its active,GTP-bound state.
Ras proteins and the proteins that regulate Ras activity have been highly conserved in evolution,and genetic analyses in Drosophila and C.elegans provided the first clues as to how receptor tyrpsine kinases activate Ras.Genetic studies of photoreceptor cell development in the Drosophila eye have been particularly informative.