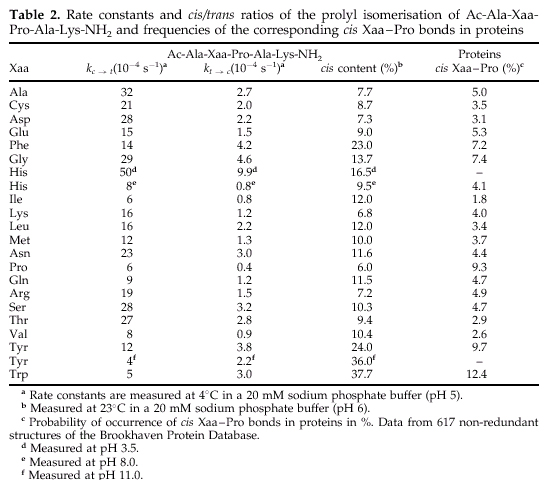
Aromatic side-chains result in slow isomerization, the rate constants decrease in the order Phe>Tyr>His>Trp.
Slow isomerization is also observed when aliphatic side-chain precede proline. The slowest reaction in this group are caused by beta-branched a.a.--Val, Ile.