|
Thrombin, a multifunctional serine
protease generated at sites of vascular injury, plays a critical role in
hemostasis and thrombosis and perhaps in inflammatory and proliferative
responses. Cellular responses
to thrombin are mediated at least in part by a family of G protein-coupled
protease-activated receptors (PARs).
PAR1, the prototypical member of this family, is irreversibly
activated upon thrombin cleavage.
Src is an important downstream effector of PAR1-mediated cellular
signaling. However, the
regulation of Src kinase activity after PAR1 activation is very
complex. In addition to being
activated and de-acitivated, Src was found to be degraded and re-expressed
after PAR1 activation. The
degradation of Src induced by PAR1 was promoted by b-arrestin2 (Kao et
al., 2006, Cell Signal. 18:1914-1923). Our preliminary results show that
Src is targeted to lysosomes for degradation after PAR1 activation. To investigate the mechanisms by
which PAR1 induces degradation of Src in lysosome, we could like to address
the following questions. Is
ubiquitin involved in PAR1-induced degradation of Src? Do ubiquitin E3 ligases and/or
lysosomal sorting machinery involved in this event? How does b-arrestin2 promote this
event? Since Src was
re-expressed after its degradation in response to PAR1 activation. We will elucidate the cellular
signaling events involved in PAR1-induced re-expression of Src and
characterize whether it is under transcriptional or translational
regulation. The re-expression
of Src might regulate PAR1-indcued gene expression, which may be related to
PAR1-promoted metastatsis of some cancer cells. We will also try to explore such
possibility.
Back
|
|
Protease-activated receptors (PARs), a
subfamily of G-protein couple receptor are activated by proteolytic
cleavage at specific sites of the extracellular domains by serine
proteases. The newly exposed
amino terminus serves as a tethered ligand, which binds to the second
extracellular loop of the receptor, to initiate signal transduction. Today, four subtypes of PARs have
been identified. They are PAR1,
PAR2, PAR3, and PAR4. PARs have
been shown to play important roles in coagulation, proliferation, survival,
inflammation and tumorigenesis.
The expression of PAR1, PAR2 and PAR4 has been reported to be
elevated in lung cancer. The increased expression of PAR1 and PAR2 might be
related to the migration and proliferation of lung cancer cells. Here, we could like to determine
whether PAR1 and PAR2 induce migration and proliferation of lung cancer
cells and the underlined molecular mechanisms. The possible PARs-mediated signal
transduction pathways leading to proliferation and migration in lung cancer
cells are shown in Fig. 1. The
involvement of G proteins and mitogen-activated protein kinases (MAPKs) in
PAR1 and PAR2-induced migration and proliferation of lung cancer cells will
be examined. Whether cancer
cell secretes unconventional proteases to activate PARs on its cell
membrane will be elucidated.
Also, whether PARs transactivate other membrane receptors, such as epidermal
growth factor receptor (EGFR), to enhance cellular responses will be
investigated.
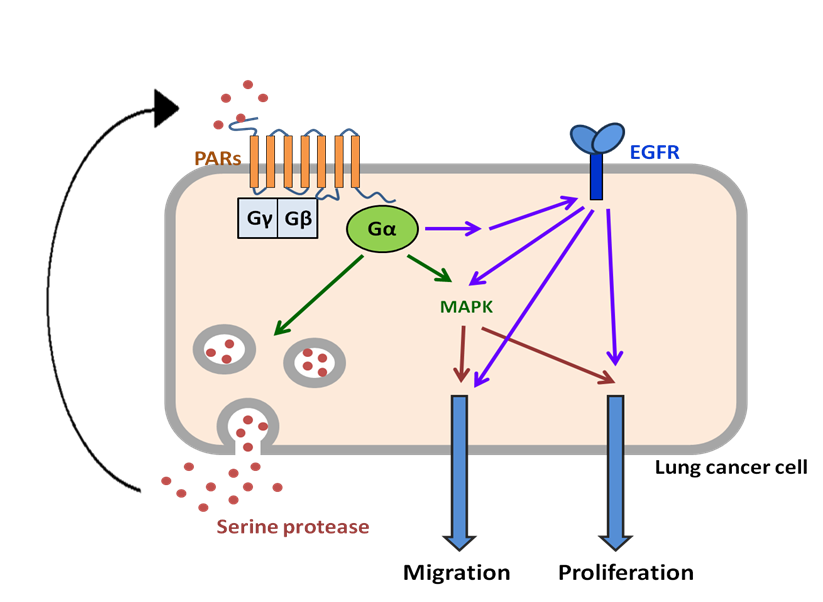
Fig. 1. Activation of protease-activated receptors involved
in migration and proliferation of lung cancer. Protease-activated
receptors (PARs) are activated by serine proteases; subsequently the
activated PARs initiate the MAPK signaling to elicit cellular responses
including migration and proliferation. In addition, transactivation of
membrane receptors such as EGFR by PARs enhances PARs-induced cellular
responses. Lung cancer cells may also secrete serine proteases and create
an autocrine loop to prolong PARs activation.
Back
|