Distance Variations between
Active Sites of
CtH+-PPase Determined by smFRET
Homodimeric H+-pyrophosphatase
(H+-PPase; EC 3.6.1.1) is a unique enzyme playing a pivotal
physiological role in pH homeostasis of organisms. This novel
enzyme supplies
energy at expense of hydrolyzing metabolic byproduct, pyrophosphate (PPi),
for H+ translocation across membrane. The functional unit for the
translocation is considered to be a homodimer. Its putative active site on each
subunit consists of PPi binding motif, Acidic I and II motifs, and several
essential residues. In this investigation, structural mapping of these vital
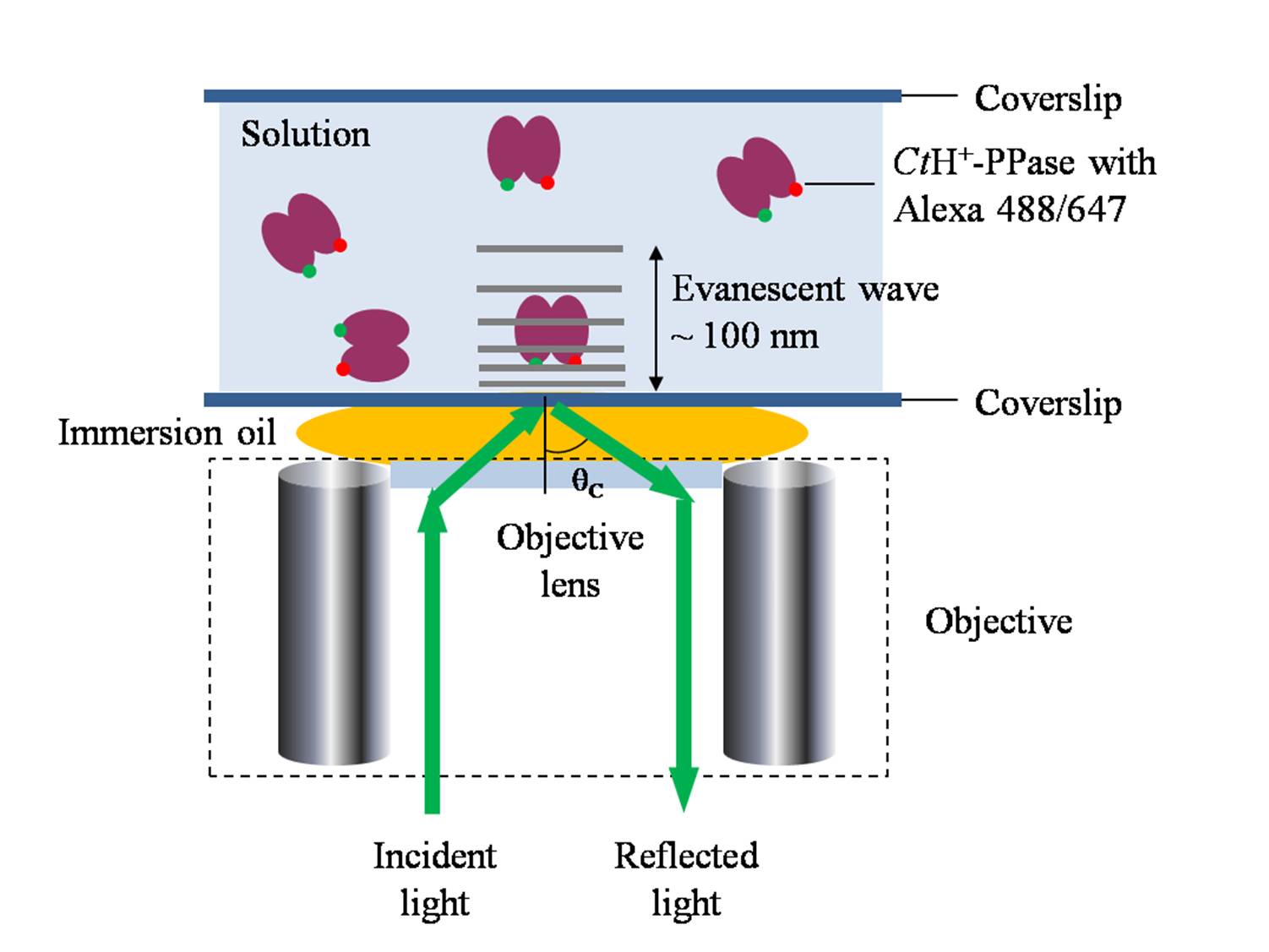
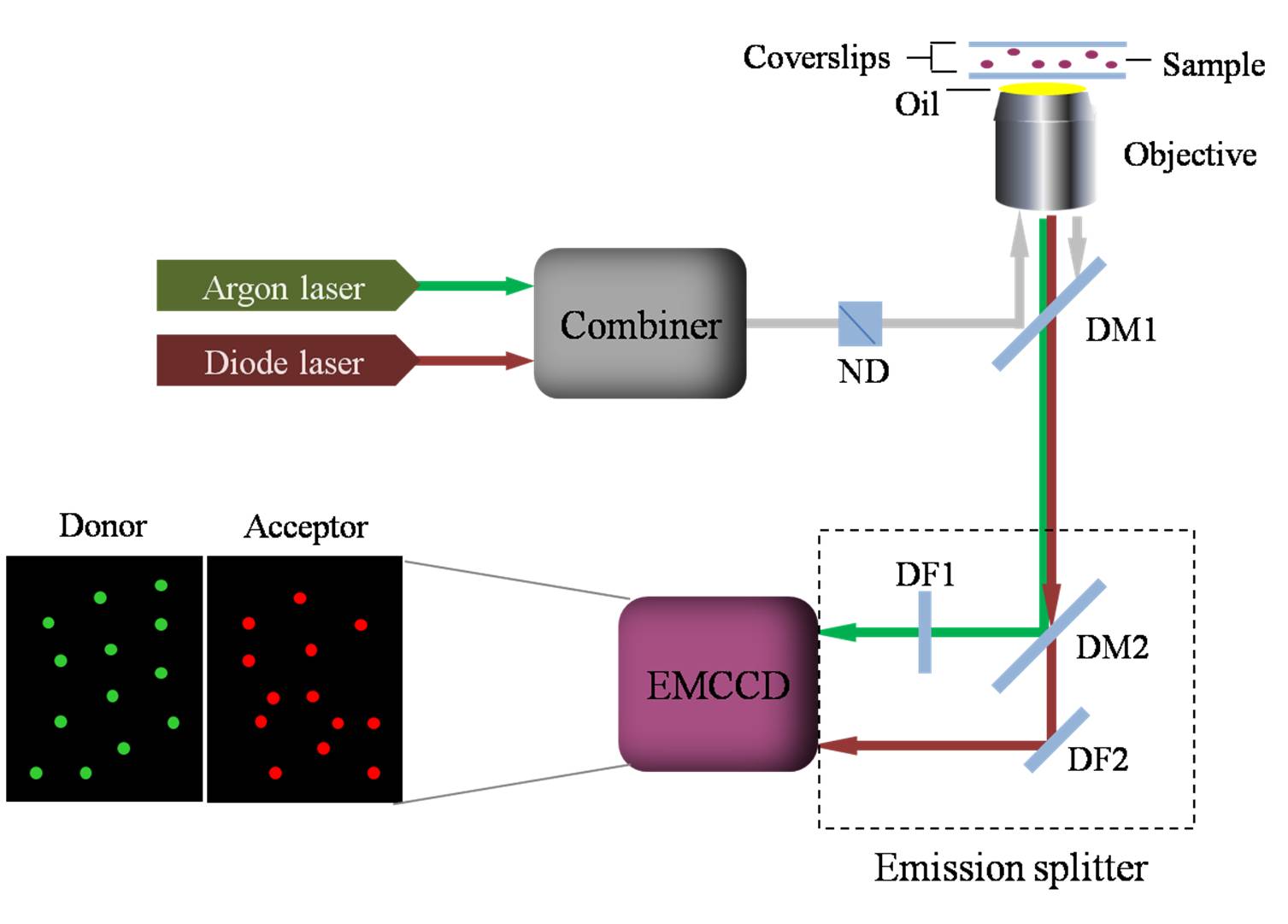
regions was primarily determined utilizing single molecule fluorescence
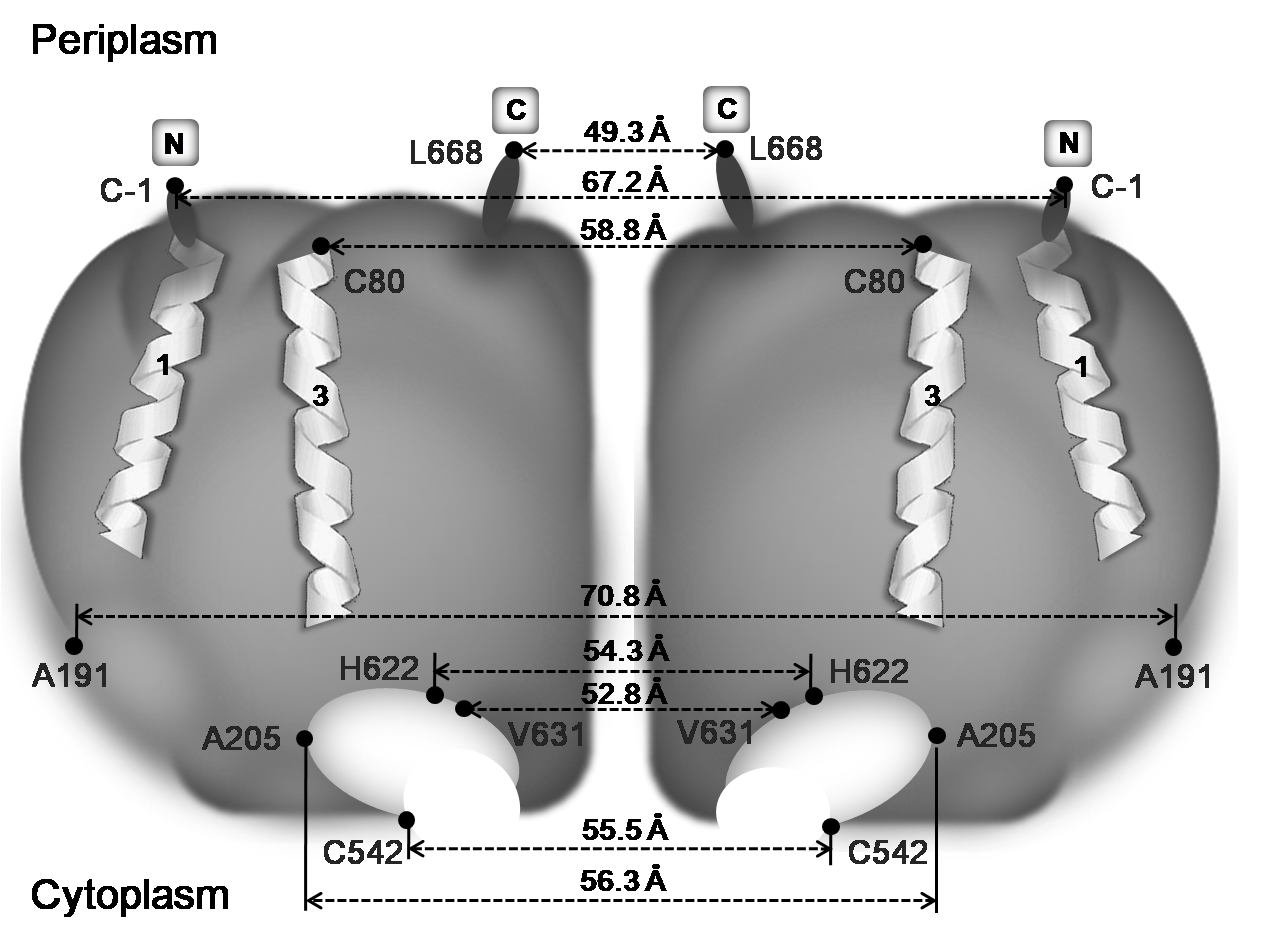
resonance energy transfer. Distances between two C termini and also two N
termini on homodimeric subunits of H+-PPase are 49.3 ± 4.0 Å and 67.2 ± 5.7 Å,
respectively. Furthermore, putative PPi
binding motifs on individual subunits are found to be relatively far away from
each other (70.8 ± 4.8 Å), while binding of
potassium and substrate analogue led them to closer proximity. Moreover,
substrate analogue but not potassium elicits significantly distance variations
between two Acidic I motifs and two H622 residues on homodimeric subunits. Taken together, this study provides the first
quantitative measurements of distances between various essential motifs, residues and putative active sites on homodimeric subunits of H+-PPase. A working
model is accordingly proposed elucidating the distance variations of dimeric H+-PPase
upon substrate binding.
Huang,
Y.T., Liu, T.H., Chen, Y.W., Lee, C.H., Chen, H.H., Huang, T.W., Hsu, S.H., Lin,
S.M., Pan, Y.J., Lee, C.H., Hsu, Ian C., Tseng, F.G., Fu, C.C., and Pan, R.L.
(2010) Distance varations between active sites of H+-pyrophosphatase
determined by fluorescence resonance energy transfer. J Bio Chem (Accepted)