1. How many different proteins does a typical cell synthesize? How many proteins of these are abundant, with over 50,000 copies present?
2. (a) Define the term "chiral centre" as applied to amino acids. (b) Which amino acids have chiral centres that are not alpha-carbon atoms?
(c) Which amino acid is this one ? Is it a D-amino acid ?
3. Please list the factors that cause the distortions of a-helices.
4.
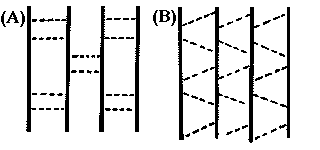
The dots in the above figure represent the hydrogen bonds between backbones. Could you identify the parallel b-sheets or antiparallel b-sheets from these hydrogen patterns?
5. What is the average length of helix in a globular protein, and why?
6. To test the role of salt bridges between oppositely charged side chains separated by three and four residues in stabilizing the a-helical conformation, two pairs of peptides of the type
(1) AEAAAKEAAAKEAAAKA
(2) AKAAAEKAAAEKAAAEA
(3) AEAAKAEAAKAEAAKA
(4) AKAAEAKAAEAKAAEA
Red: negatively charged residues
Blue: positively charged residues
were compared (S. Marqusee and R. L. Baldwin, Proc. Natl. Acad. Sci. USA 84, 8898-8902, 1987). The a-amino and a-carboxyl groups were blocked with acetyl and amide groups, respectively. How would the first and second peptides of each pair be expected to differ in their helicity? What's the reasons for these differences?
7. (a) What is the helix capping box ? (b) How can you prove it experimentally ?
8. Please predict the secondary structure of the following peptide and also describe the method you use.
SEDLANQVEKLTKNFHRVNESIAGT
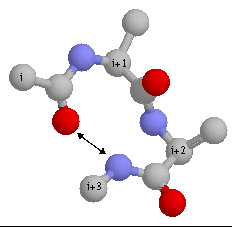
9. Which type of turn is this? (type I, type II, type III, type I' .....)
Which residue is often found in i+1 position of this type of turn?