The Rana
catesbeiana Ribonuclease (RC-RNase) Inhibitor (RC-RI)
(July 3 ~August 27, 2000)
Ls02G 871616 Wai-Li Hong
Institute of Biomedical Sciences, Academia Sinica, Taipei 115, Taiwan,
Dr.You-Di
Liao(廖有地老師)
ABSTRACT
Ribonuclease ( RC-RNase) is abundant in the oocytes
and early embryos of bullfrog (Rana
catesbeiana). However, eggs derived from ribonuclease abundant
oocytes are able to develop into tadpoles and the oocytic extracts are transcriptionally
active in vitro. It is proposed that RC-RNase play roles during embryogenesis
and their ribonucleolytic activities are well regulated to prevent damages to
cellular RNAs.
Ribonucleases with antitumor activity are mainly found in the oocytes and embryos
of frogs, but the role of these ribonucleases in frog development is not clear.
Moreover, most frog ribonuclease genes have not been cloned and characterized.
In the present study, a group of ribonucleases were isolated from Rana catesbeiana
(bullfrog). These ribonucleases in mature oocytes, namely RC-RNase,
RC-RNase 2, RC-RNase 3,
RC-RNase 4, RC-RNase 5
and RC-RNase 6, as well as liver-specific ribonuclease
RC-RNase L1, were purified by column chromatographs
and detected by zymogram assay and western blotting.However, wejust know a few
about RC-RI.
INTRODUCTION
Ribonucleases are widely found in living organisms and
have been proposed to function in RNA metabolism and gene expression. Several
abundant ribonucleases have been isolated from organs of various animals and
have been well characterized. For example, various kinds of ribonuclease have
been purified from bovine organs, e.g. pancreas, liver, kidney, brain, and seminal
fluids. RNase A, from bovine pancreas, has been extensively characterized and
is widely used in molecular biology. The occurrence of several homologous ribonucleases
in different organs of the same animal suggests the existence of a family of
homologous genes regulated in a tissue-specific fashion. Although these ribonucleases
are abundant and well characterized biochemically, their biological significance
is still not clear. Recently several proteins with known biological functions
were found to have intrinsic ribonucleolytic activity. For example, angiogenin
from humans possesses both angiogenesis and ribonucleolytic activities. Eosinophil-derived
neurotoxin and eosinophil cationic protein from humans exert both neurotoxicity
and ribonucleolytic activity. Bovine seminal ribonucleases, a dimer made up
of two identical 124 amino acid subunits, exerts both antitumor and ribonucleolytic
activity. The ribonucleases from frog oocytes exert antitumor activity as well
as ribonucleolytic activity, e.g. onconase from Rana pipiens and RC-RNase from
Rana catesbeiana.
The RC-RNase gene rcr is cloned from bullfrog liver and expressed in
Escherichia coli. The recombinant RC-RNase has same amino acid sequence,
specific activity, substrate specificity and antigenicity as those of native
RC-RNase from bullfrog oocytes. Amino acid residues for ribonucleolytic activity
are similar to those of ribonuclease superfamily genes including bovine pancreatic
RNase A, human angiogenin and human eosinophil-derived neurotoxin. RC-RNase
is cytotoxic to several tumor cells rather than normal cells. The morphologies
of RC-RNase-treated cells are examined by light microscope, SEM and TEM. The
entry pathway of RC-RNase to tumor cells is under investigation. The ribonucleolytic
activity of RC-RNase is essential for its cytotoxicity against tumor cells.
Three distinct properties are found in the frog RNases.
First, the substrate specificity of frog RNase
is pyrimidine-guanine and that of mammalian RNase is pyrimidine-adenine. Second,
RNase from the frog is resistant to RNase inhibitor from human placenta, whereas
mammalian RNase is susceptible to the inhibitor. Third,
the RNases of frogs with antitumor activity are found in oocytes, e.g. bullfrog
RC-RNase, whereas most of mammalian RNases with cytotoxic activity are found
in eosiniphils. The interactions of RNase inhibitor (RI), a 50-kDa protein which
is isolated from human placenta, with the various "14-kDa members of the pancreatic
RNase superfamily provide a particularly rich and intriguing system for investigating
the important problem of protein-protein recognition. RI complexes (e.g. RI-RNase
A complex [Kobe, B. & Deisenhofer, J. (1995) Nature (London) 374, 183-186])
are among the tightest on record: dissociation constants for the seven ligands
whose binding has been kinetically characterized extend from 10-13 to less
than 10-15M. The high affinities of RI for all of its ligands is remarkable
in view of the modest degree of sequence identity that these protein share.
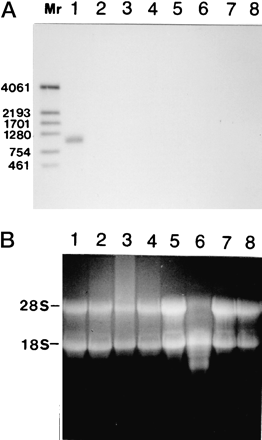
Fig. 1. Northern blotting analysis of RC-RNase mRNA expression
in bullfrog tissues/organs.
A, 20 ?g of total RNA from different tissues/organs of a single bullfrog. The
RNAs were probed with biotin-labeled RC-RNase DNA fragment. Lane 1, liver;
lane 2, pancreas; lane 3, kidney;
lane 4, stomach; lane 5, intestine;
lane 6, ovary; lane 7, spleen;
lane 8, liver from a male bullfrog.
B, total RNAs from different tissues/organs were stained
by ethidium bromide. The amounts of RNA loaded and sample alignment were
identical to A
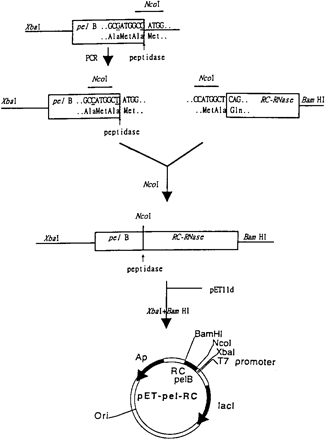
Fig. 2.Construction of expression vector pET-pel-RC
MY
JOB ...
1.Use partial regions of the amino acid
sequence to design the primers of RT-PCR.
2.Find a optimal system to express RC-RI and.
3.Purification of RC-RI.
MATERIALS
AND METHODS
Frogs
-Native bullfrogs (Rana catesbeiana)
were obtained from a local frog farm and housed at 25℃.
Isolation
of Total RNA from tissue -Total RNA
from the liver and kidney of R. catesbeiana was isolated by two ways:
I. Traditional method
1. 0.5g of tissue quickly frozen in liquid nitrogen.
2. Ground by mortar E Add 2.5ml GTC (guanidinium
thiocyanate) and mix with the powder.
3. Pour sample mixture into 15-ml falcon tube.
4. Add 2.5ml GTC and liquidlize at RT.
5. Add 5ml water-saturated phenol.
6. Add 1ml (2/10 volume) CIAA (chloroform isoamyl alcohol).
7. Add 0.5ml (1/10 volume) 2M NaOAc (pH4.5) and Mix sample
violated.
8. Centrifugation at 4000rpm for 10 or 15 min.
9. Save supernatant and add equal volume of IPA (isopropyl
alcohol).
10. Mix and keep at -20℃ for I hour.
11. Centrifugation at 4000rpm for 10 or 15 min.
12. Dissolved pellet in 2ml GTC.
13. Add 2× volume of ethanol and 1/10 volume of 3M NaOAc
(pH5.4).
14. Mix and keep at -20℃ for I hour.
15. Centrifugation at 400rpm for 10 or 15 min.
16. Wash pellet with 70% ethanol.
17. Dissolve pellet in DEPC H2O
(20ml).
18. Read OD 260/280 by spectrophotometer.
II. RNAzol method
1. Homogenize tissue samples with RNAzol B (2 mL per
100 mg tissue)
2. Add 0.2 mL chloroform per 2 mL of homogenate, shake
vigorously for 15 seconds (do not vortex) and let them stay on ice for 5 min.
3. Centrifuge the suspension at 12,000 g (4℃) for 15
min.
4. Transfer the aqueous phase to the fresh tube, add
an equal volume of isopropanol and store the samples for 15 min at 4℃.
5. Centrifuge samples for 15 min at 12,000 g (4℃).
6. Remove the supernatant and wash the
RNA pellet once with 75% ethanol by vortexing and subsequent centrifugation
for 8 min at 7,500 g (4℃ or -20℃).
7. Dry the RNA pellet but do not let the pellet dry completely,
as it will greatly decrease its solubility.
8. Dissolve the RNA pellet in DEPC (Diethylpyrocabonate).
Quantify
the RNA- The total RNA we isolated from the
frog organs should have to be sure high quality.We can run formaldehyde agarose
gel(~1%) to check rRNA, or to test the OD260 and OD280
value by spectrophotometer.
RT-PCR
and RNA cloning of RC-RI Gene-To clone the
RC-RI gene, degenerate primers for partial regions of the amino acid sequence
of the RC-RI were designed to amplify a about 3300-base pair RNA fragment. The
cycle protocol for PCR was as follows: 3 cycles of 94℃ for 1 min, 42℃ for 1
min, and 72℃ for 1 min followed by 30 cycles of 94℃ for 30 s, 45℃ for 1 min,
and 72℃ for 50 s.

Rana
catesbeiana
科 別:赤蛙科
繁殖水域:大型之池塘或開闊水域以及公園及學校內之池塘。
卵與蝌蚪:卵散布於靜止水面,蝌蚪體型巨大,全長可超過lOOmm,且有越冬
現象。
成蛙外型:為大型蛙類,體長約120~150mm,外表以綠色為主,或參雜以棕 色或灰棕色之花紋,常現出如迷彩裝般之花紋。
習 性:牛蛙為外來種蛙類,主要為人們飼養之食用蛙,野外之牛蛙大多為 逃逸或人們棄置之個體。其嗚聲大而低沈。
分 佈: 在野外沒有固定族群,但是偶而會在公園或校園的大型池塘畔,見 到被放養的牛蛙,以台大醉月湖及陽明山的湖底和大屯自然公園較常見。


