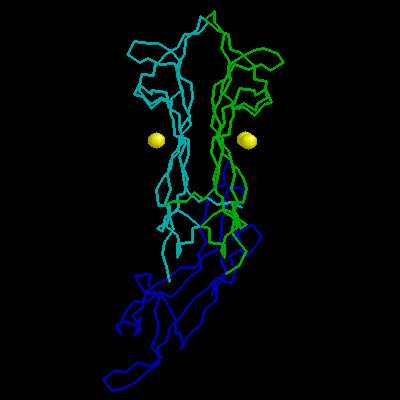
 | Beta-Nerve Growth Factor |
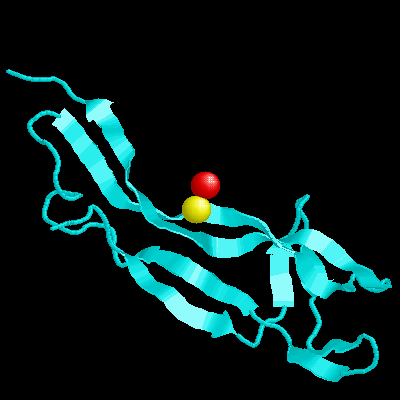
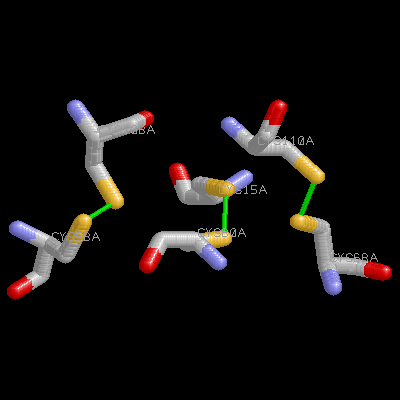
 Beta-nerve growth factor (beta-NGF) is a member of an expanding family of neurotrophic factors (including brain-derived neurotrophic factor and the neurotrophins) that control the development and survival of certain neuronal populations both in the peripheral and in the central nervous systems. Its biological effects are mediated by a high-affinity ligand-receptor interaction and a tyrosine kinase signalling pathway. A potential use for NGF and its relatives in the treatment of neurological disorders such as Alzheimer's disease and Parkinson's disease requires an understanding of the structure-function relationships of NGF.Murine beta-nerve growth factor (beta NGF) is a 118 amino acid residue polypeptide which, as a functional dimer, plays an important role in the survival and development of certain neuronal populations. The structure of the bis-desocta1-8 form of murine beta NGF has been determined in two different crystal modifications using X-ray methods. The P2(1)2(1)2(1) structure was solved by molecular replacement using the C2 structure as a search model and was refined to a crystallographic residual of 19.7% at 2.8 A resolution. This crystal form contains two monomers per asymmetric unit, again arranged as a non-crystallographic 2-fold-related dimer. The N and c termini are also variably defined. The core of each of the five monomers, which forms a cysteine knot motif, is very similar in all structures. Also, the dimer structures are very similar to one another, whether the monomers are related by crystallographic or non-crystallographic symmetry. However, three of the four loop regions that extend from the core of each monomer display substantial variability in conformation, even between monomers of the same dimer. This structural variability in the putative receptor binding regions suggests that structural malleability might be important in allowing the ligands to bind to different receptors with different affinities.A clustering of positively charged side chains may provide a complementary interaction with the acidic low-affinity NGF receptor. The structure provides a model for rational design of analogues of NGF and its relatives and for testing the NGF-receptor recognition determinants critical for signal transduction.
Beta-nerve growth factor (beta-NGF) is a member of an expanding family of neurotrophic factors (including brain-derived neurotrophic factor and the neurotrophins) that control the development and survival of certain neuronal populations both in the peripheral and in the central nervous systems. Its biological effects are mediated by a high-affinity ligand-receptor interaction and a tyrosine kinase signalling pathway. A potential use for NGF and its relatives in the treatment of neurological disorders such as Alzheimer's disease and Parkinson's disease requires an understanding of the structure-function relationships of NGF.Murine beta-nerve growth factor (beta NGF) is a 118 amino acid residue polypeptide which, as a functional dimer, plays an important role in the survival and development of certain neuronal populations. The structure of the bis-desocta1-8 form of murine beta NGF has been determined in two different crystal modifications using X-ray methods. The P2(1)2(1)2(1) structure was solved by molecular replacement using the C2 structure as a search model and was refined to a crystallographic residual of 19.7% at 2.8 A resolution. This crystal form contains two monomers per asymmetric unit, again arranged as a non-crystallographic 2-fold-related dimer. The N and c termini are also variably defined. The core of each of the five monomers, which forms a cysteine knot motif, is very similar in all structures. Also, the dimer structures are very similar to one another, whether the monomers are related by crystallographic or non-crystallographic symmetry. However, three of the four loop regions that extend from the core of each monomer display substantial variability in conformation, even between monomers of the same dimer. This structural variability in the putative receptor binding regions suggests that structural malleability might be important in allowing the ligands to bind to different receptors with different affinities.A clustering of positively charged side chains may provide a complementary interaction with the acidic low-affinity NGF receptor. The structure provides a model for rational design of analogues of NGF and its relatives and for testing the NGF-receptor recognition determinants critical for signal transduction.  |
|
 |
|
 |
|


 Mail to me:b821605@life.nthu.edu.tw
Mail to me:b821605@life.nthu.edu.tw