The Crystal Structure of Gelsolin
Leslie D.Burtnick,et al.
Cell Vol. 90, 661-670, August 22, 1997
Summary and Introduction
¡@¡@The crystall structure of plasma gelsolin without Ca+ has been determined by this group. Gelsolin is a protein participates in actin capping, severing, and nucleating. These actions are important in cell mobility(intracellular form) and plasma F-actin cleaning(plasma form).It has been known that there are six domains in only one polypeptide of gelsolin. And Data from limited proteolytic digestion shows that :
- S1 binds G-actin in the abscent of Ca+, and the resulting complex can cap actin filament
- S4-S6 constitute a second, Ca+-dependent, actin monomer-binding fragement that competes for the same binding on actin as S1
- S2 contains a Ca+ independent F-actin binding site.
- Nucleation of actin filaments can be achieved with S2-S6
- F-actin severing capability is contained in S1-S3
By the structure solved by the group and the data has been known, they propose models of how gelsolin works in capping, severing, and nucleating.
Results
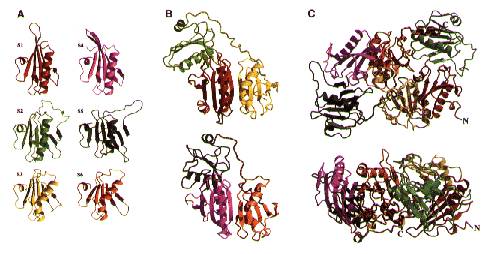 The Structure of Gelsolin
The Structure of Gelsolin
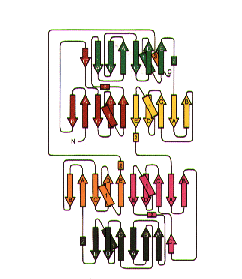 The Topology of Gelsolin
The Topology of Gelsolin
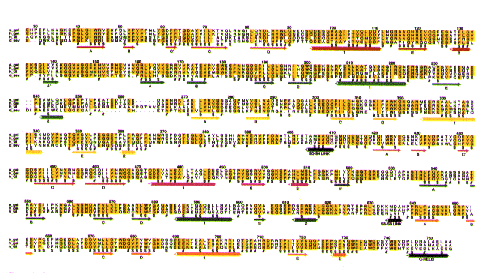 Sequence Comparisons of Gelsolin
Sequence Comparisons of Gelsolin
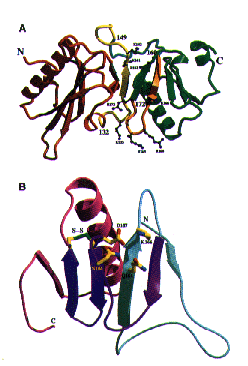 How mutation at Asp187 causes FAF
How mutation at Asp187 causes FAF
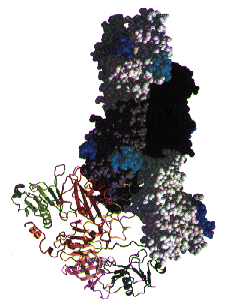 A model for Actin Capping
A model for Actin Capping
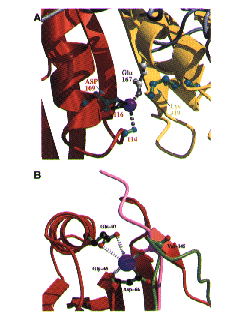 Ca+ control of Gelsolin Function
Ca+ control of Gelsolin Function
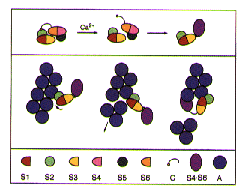 A Schematic Model of Ca+ controlled Severing of F-Actin by Gelsolin
A Schematic Model of Ca+ controlled Severing of F-Actin by Gelsolin
 The Topology of Gelsolin
The Topology of Gelsolin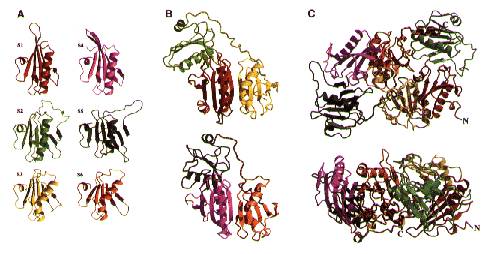 The Structure of Gelsolin
The Structure of Gelsolin Sequence Comparisons of Gelsolin
Sequence Comparisons of Gelsolin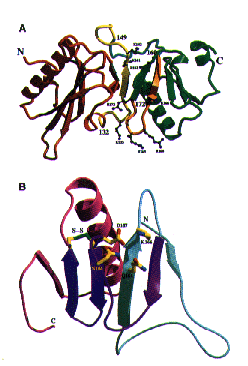 How mutation at Asp187 causes FAF
How mutation at Asp187 causes FAF Ca+ control of Gelsolin Function
Ca+ control of Gelsolin Function A Schematic Model of Ca+ controlled Severing of F-Actin by Gelsolin
A Schematic Model of Ca+ controlled Severing of F-Actin by Gelsolin