Institute of Molecular and Cellular Biology & Department of Life Sciences
Dr. Oliver Wagner, Professor
王歐力教授 |
BRIEF INTRODUCTION
My lab is interested in the regulation of organelle trafficking and molecular motors in the nervous system. We are using the model organism C. elegans with a fully sequenced genome.
In the image below, the nervous system of such a worm (1 mm in length) has been made visible by expressing a neuronal specific molecular motor (KIF1A/UNC-104). When zooming in, details of nerves and nerve bundles are evident including single cell bodies and long axonal extensions.
Time-lapse imaging reveals the bidirectional motility pattern of KIF1A/UNC-104. However, only little is known about the signals that trigger stops, starts and reversals of these molecular motors.
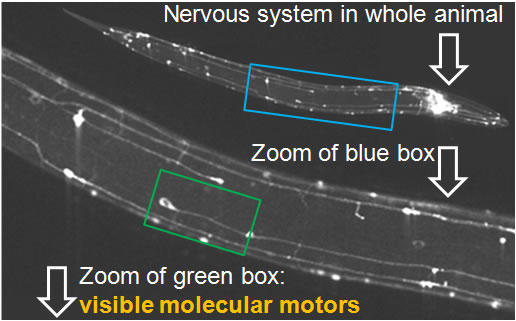
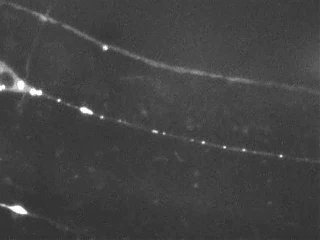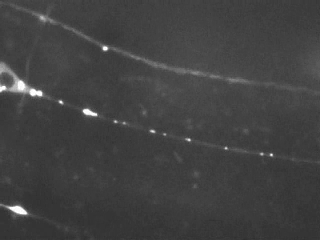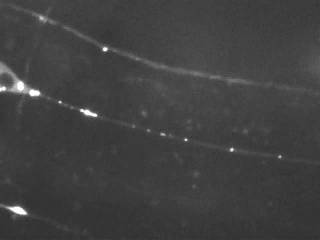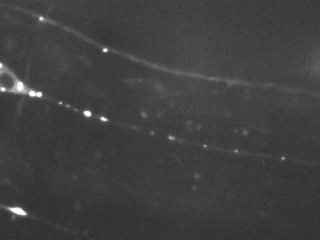
The nervous system of C. elegans is simple, yet, excellent for studying axonal transport due to the accessibility of very long axons (up to 500 microns) and the high homology of neuronal proteins compared to mammals.
Studying the regulation of motors becomes increasingly important as several motors are involved in various neurodegenerative diseases:
Neurodegenerative disease |
Involved molecular motors (and their adaptors) |
• Alzheimer’s disease
• Huntington’s disease
• Spastic Paraplegia |
Kinesin-1 (with adaptors APP and Huntingtin) |
• Charcot-Marie-Tooth disease
• Multiple Sclerosis
• Senile Dementia |
Kinesin-3 |
• ALS
(amyotrophic lateral sclerosis)
• Lissencephaly
• Lower motor neuron disease |
Dynein (with adaptors dynactin and LIS1) |
Source: Hirokawa, N., et al., 2010. Neuron. 68, 610-38
One of the basic questions regarding the regulation of molecular motors is how they recognize their cargo and how their motility is controlled. Theories exist that molecular motors are activated by phosphorylation or, more interestingly, by binding to the cargo itself. Recently, we have identified two (active zone) proteins (SYD-2 and LIN-2) that differentially regulate kinesin-3 motor motility (Wagner et al., PNAS, 2009; Wu et al., Traffic, 2016). In a follow-up study, we identified how a phosphatase regulates intramolecular folding of SYD-2 to activate kinesin-3 (Shanmugam at al., MBoC, 2020). We have intensively dissected kinesin-3/dynein interactions (Chen et al., JNS, 2019) as well have characterized tau (Tien et al., Neurobiol. Dis, 2011) and neurofilament (Bhan et al., Traffic, 2019) homologs in C. elegans. We are also interested in intraflagellar transport (IFT), and have identified and characterized two novel phosphatases regulating IFT and ciliogenesis in C. elegans (Shanmugam et al., MCB, 2018). Recently, we identified a neuronal C. elegans intermediate filament that acts to stabilize mitochondria on their long-range axonal travels (Barmaver et al., Traffic, 2022).
Our research has been featured on the covers of Traffic (2022, Issue 23, Vol 5), Mol Biol Cell (2020, Issue 15) and Mol Cell Biol (2018, Issue 7, Vol 38):


Contact Information:
Oliver I. Wagner, PhD
Professor
National Tsing Hua University
Institute of Molecular and Cellular Biology
Department of Life Science
101, Sec. 2, Kuang Fu Road
Hsinchu 30013
Taiwan (R. O. C.)
Tel: +886-3-574-2487
Fax: +886-3-571-5934
Email: owagner(at)life.nthu.edu.tw (please CC to wagner.nthu(at)gmail.com)
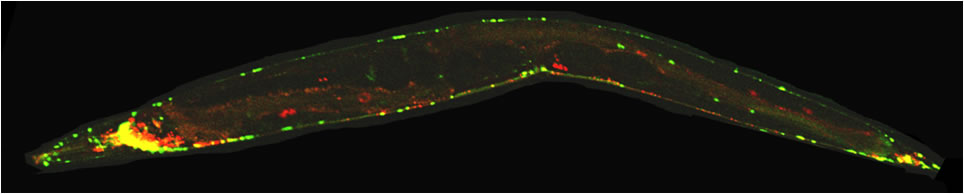
*The title image shows a worm double expressing a red fluorescent synaptic marker and green fluorescent molecular motors. The head of the worm faces to the left and the tail to the right. Head ganglia reveal strong colocalization between motor and cargo.